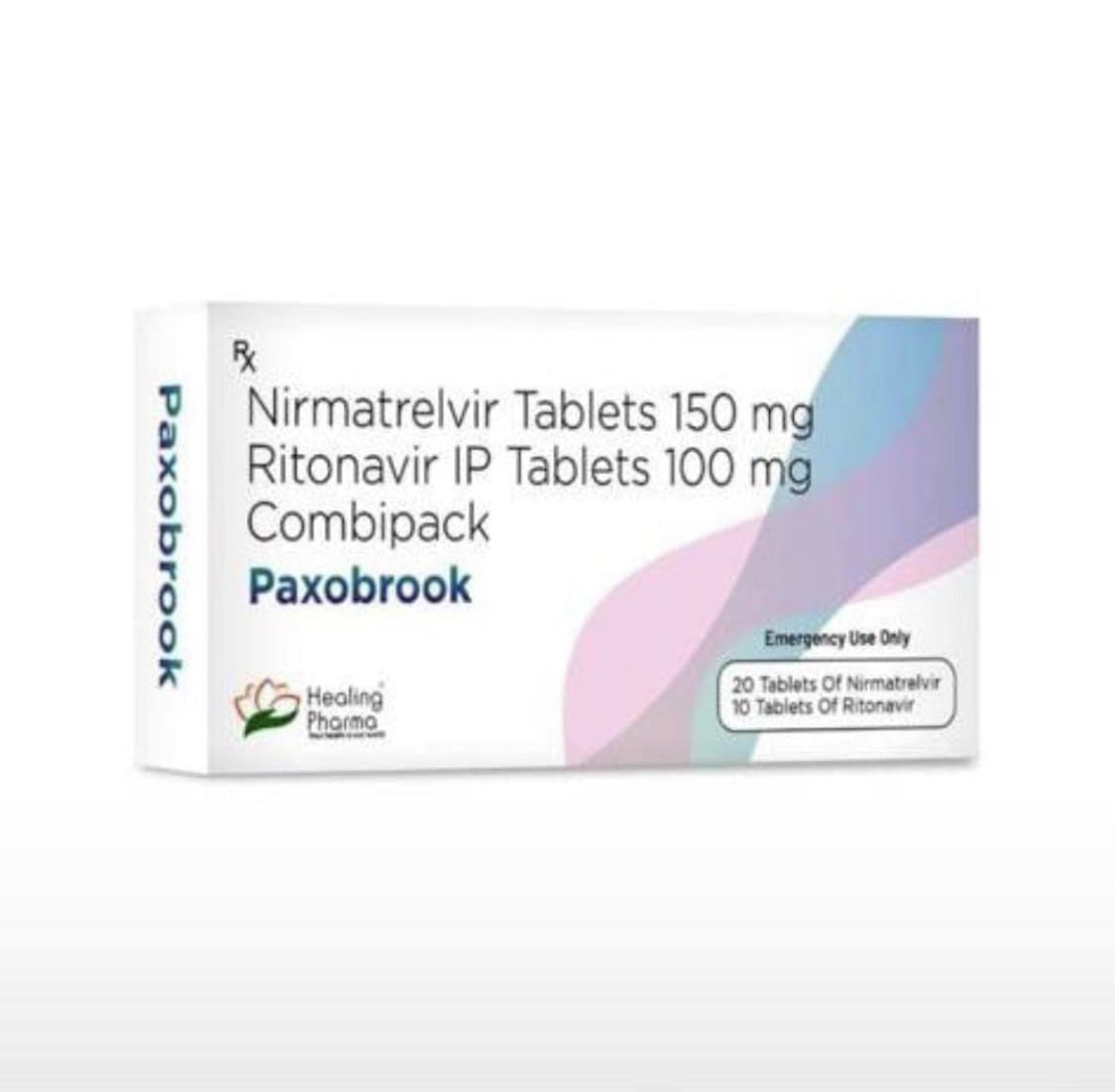
Paxobrook
$200.00 – $875.00Price range: $200.00 through $875.00
SKU : N/A
Category : Generic Paxlovid
Paxobrook is an antiviral medication used to treat mild to moderate COVID-19 in adults, helping reduce symptoms and prevent severe illness when taken early.
Paxobrook is an oral antiviral medication formulated to combat COVID-19 infections in adults and certain adolescents who are at high risk for progressing to severe illness. Designed with a combination of two active pharmaceutical ingredients — Nirmatrelvir and Ritonavir — Paxobrook works by inhibiting viral replication and enhancing antiviral potency, giving patients a better chance at faster recovery and reduced hospitalization.
As new variants of SARS-CoV-2 continue to emerge, effective therapeutic tools like Paxobrook remain essential in the global fight against COVID-19. Its ease of use, oral route of administration, and impressive efficacy profile make it a practical solution for high-risk individuals in outpatient settings.
What is Paxobrook?
Paxobrook is a co-packaged antiviral treatment used to reduce the severity and progression of COVID-19. It is designed for use in patients with mild to moderate symptoms who are not hospitalized, but who are at high risk for developing severe illness due to age or underlying health conditions.
Each course of Paxobrook treatment consists of a five-day regimen involving:
-
Nirmatrelvir, which directly targets the coronavirus by inhibiting its main protease enzyme (3CLpro).
-
Ritonavir, which serves as a pharmacokinetic booster that slows down the breakdown of Nirmatrelvir, prolonging its effectiveness in the body.
Together, this dual-action therapy ensures that viral replication is suppressed, helping patients recover faster and reducing the risk of complications or hospitalization.
Mechanism of Action
Paxobrook’s effectiveness hinges on its dual mechanism:
-
Nirmatrelvir: This component is a protease inhibitor, which blocks the SARS-CoV-2 main protease enzyme. This enzyme is essential for viral replication; without it, the virus cannot process its polyproteins, making it incapable of producing functional viral particles.
-
Ritonavir: Though originally developed as an HIV drug, Ritonavir is not used here for its antiviral activity. Instead, it acts as a CYP3A inhibitor, preventing the rapid breakdown of Nirmatrelvir in the liver, thereby boosting its plasma concentration and prolonging its antiviral action.
The combined effect results in reduced viral load, mitigated symptoms, and a lower likelihood of hospitalization or death in high-risk patients.
Indications
Paxobrook is prescribed for the treatment of mild-to-moderate COVID-19 infection in:
-
Adults (18+ years)
-
Adolescents (12–17 years weighing at least 40 kg)
-
Individuals at high risk for progressing to severe COVID-19, including hospitalization or death
Risk factors include:
-
Age above 60
-
Chronic illnesses (diabetes, hypertension, obesity, heart/lung disease)
-
Immunocompromised status
-
Cancer, HIV, organ transplant recipients
Dosage and Administration
Typical Paxobrook Dose
-
Two 150 mg tablets of Nirmatrelvir
-
One 100 mg tablet of Ritonavir
-
Taken twice daily for 5 days
Total Daily Dose:
-
Morning: 2 Nirmatrelvir + 1 Ritonavir
-
Evening: 2 Nirmatrelvir + 1 Ritonavir
Administration Notes:
-
Can be taken with or without food
-
Must be started within 5 days of symptom onset
-
Complete all doses even if symptoms improve early
Missed Dose:
-
If a dose is missed and it’s less than 8 hours late, take it as soon as possible
-
If more than 8 hours have passed, skip the missed dose and continue with the next scheduled dose
Who Should Use Paxobrook?
Paxobrook is suitable for:
-
Recently diagnosed COVID-19 patients with mild to moderate symptoms
-
Individuals at increased risk of complications
-
Non-hospitalized patients seeking early intervention to avoid deterioration
Benefits of Paxobrook
-
Reduces hospitalization rates among high-risk individuals
-
Shortens symptom duration and speeds up recovery
-
Can be used at home, eliminating the need for IV treatment or hospitalization
-
Convenient oral tablet form
-
Well-suited for use in outpatient or telehealth settings
Contraindications and Precautions
Contraindications:
-
Known hypersensitivity to Nirmatrelvir, Ritonavir, or any excipients
-
Concurrent use of medications that are highly dependent on CYP3A for clearance (due to serious drug interactions)
Use with Caution:
-
Patients with severe liver or kidney impairment
-
Pregnant or breastfeeding women (only if clearly needed)
-
Pediatric patients under 12 years or weighing less than 40 kg
Possible Side Effects
Paxobrook is generally well tolerated, but some individuals may experience mild to moderate side effects.
Common Side Effects:
-
Altered taste (metallic or bitter taste)
-
Diarrhea
-
Muscle aches
-
Headache
-
Nausea
Less Common Side Effects:
-
Elevated liver enzymes
-
High blood pressure
-
Allergic reactions (rash, itching, swelling)
Serious Reactions (Rare):
-
Hepatotoxicity
-
Bradycardia
-
Severe allergic or anaphylactic reactions
Seek immediate medical help if signs of allergic reaction or serious side effects occur.
Drug Interactions
Ritonavir has a high potential for drug interactions due to its CYP3A inhibition. Always inform your doctor about all medications, supplements, or herbal remedies you are taking.
Do Not Combine With:
-
Certain antiarrhythmics (e.g., amiodarone)
-
Sedatives (e.g., midazolam, triazolam)
-
Ergot derivatives
-
Certain statins (e.g., simvastatin, lovastatin)
-
Rifampin (strong CYP3A inducer)
Your healthcare provider may adjust or temporarily discontinue medications to allow safe use of Paxobrook.
Storage Instructions
-
Store at room temperature between 15°C and 30°C
-
Keep blister packs sealed until use
-
Protect from moisture and heat
-
Keep out of reach of children and pets
Frequently Asked Questions (FAQs)
Q1: Can Paxobrook be used for COVID-19 prevention?
A: No. Paxobrook is not approved for pre-exposure or post-exposure prophylaxis. It is used only after a confirmed COVID-19 diagnosis.
Q2: When should Paxobrook be started?
A: As early as possible, ideally within 5 days of symptom onset for best effectiveness.
Q3: Can vaccinated individuals take Paxobrook?
A: Yes. Even vaccinated individuals at high risk can benefit from Paxobrook if they develop COVID-19.
Paxobrook offers a powerful, convenient, and effective solution for the treatment of mild to moderate COVID-19 in high-risk individuals. By combining Nirmatrelvir, a targeted antiviral, with Ritonavir, a booster that enhances its longevity, Paxobrook empowers patients to manage their infection at home and avoid complications that could lead to hospitalization or worse.
When taken early, Paxobrook can dramatically alter the course of the illness — making it an essential option in the early outpatient management of COVID-19. Despite its potential for drug interactions, proper medical oversight can ensure safe and effective use in most eligible patients.
| Quantity Value |
120 Tablet/s ,150 Tablet/s ,30 Tablet/s ,60 Tablet/s ,90 Tablet/s |
|---|
FREE STANDARD SHIPPING on orders of $149 or more
How to get FREE Standard Shipping:
Ship to an address within the United States (including U.S. territories)
Place your online order of $149 or more* – after promotions and before tax are applied
Use “Standard” shipping option at checkout
Orders will be shipped within 24 hours of order placement*, pending verification of billing information and shipping method selected. International orders and orders containing gift cards or items that are not currently in stock will be processed as quickly as possible.
Excludes all orders placed on major US holidays (Memorial Day, Independence Day, Labor Day, Thanksgiving Day, Christmas Day and New Year’s Day).
Orders for states outside of the continental U.S. (including those of $149 or more) may be subject to a delivery surcharge. This will be noted in the order summary based on your shipping address at checkout.









Reviews
There are no reviews yet.